For this assignment, we used milk, food coloring, and dish soap. Soap breaks the milk’s surface tension, causing the food coloring to spread.
The Effect of Soap on the Surface Tension of Milk
Cooper Wyrick
Team Second
MCEN 4151-001
October 29, 2025
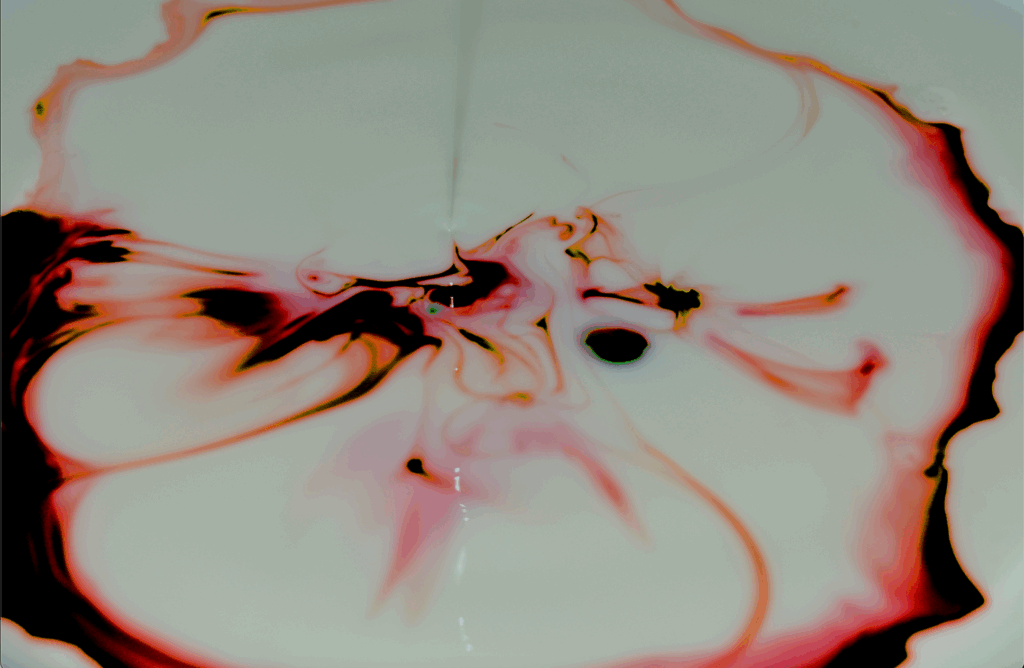
This image was created for the Team Second assignment, which was designed to get students to collaborate and investigate the artistic and scientific aspects of fluid flow. The purpose of this project was to visualize and analyze the breakage of surface tension in milk when a surfactant, dish soap, was introduced. My intent was to capture the moment when soap disrupted the surface forces of milk, causing food coloring to rapidly spread outward in colorful patterns. The goal was to document how molecular forces create visible motion on a liquid surface and explore the physics that make this popular phenomenon possible. This experiment was performed without additional assistance.
The experiment was performed using a small, shallow white plate with a diameter of approximately 20 cm and a depth of 2.5 cm. The plate was filled with 2 % milk to a depth of about 1 cm. Several drops of 365 brand red food coloring were added near the center of the surface, followed by a single drop of Dawn dish soap from its container. The schematic diagram below shows the setup.
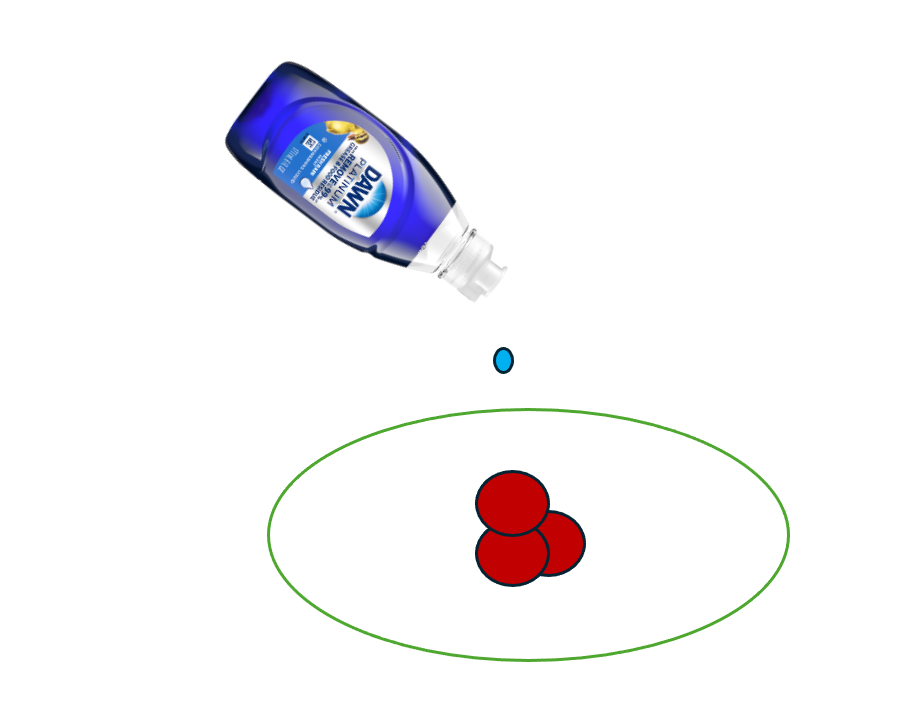
Figure 1: Schematic of Setup
When the soap contacted the milk, the surfactant molecules broke the forces at the surface by breaking down the hydrogen bonds between water molecules and dispersing the milk’s fat molecules. The reduction in surface tension caused the colored milk to flow outward, creating a swirling motion and circular pattern. The primary forces acting on the fluid included surface tension gradients, known as the Marangoni effect, as well as viscous drag seen in the thin streaks of food dye (“LECTURE 4”). The velocity of dye dispersion was estimated at approximately 1 m/s based on visual inspection. The characteristic length scale was taken as the radius of the initial color front, estimated at about 2 cm, and the kinematic viscosity of milk was approximated as 1.0 × 10⁻⁶ m²/s, similar to that of water. Using these values, the Reynolds number was calculated as Re = (UD)/ν = (1 m/s)(0.02 m)/(1.0×10⁻⁶ m²/s) = 2.0×10⁴. This relatively high Reynolds number indicates that the flow was turbulent, which aligns with the swirling behavior observed in the flow. The motion was driven primarily because the soap changed the molecular forces at the surface, and those differences pulled the liquid outward, which is a form of Marangoni convection. Similar behavior has been documented by researchers at the University of California, Santa Barbara, who showed how soap-driven Marangoni flows can propel dye through a maze by following paths of least surface tension resistance (“A Scientific”).
The visualization technique involved the interaction between milk, dye, and soap. The materials used included Kroger brand 2% milk, 365 brand red food coloring, and Dawn dish soap as the surfactant dropped from 10 cm above the milk’s surface. The experiment was performed at room temperature, approximately 21°C, under dim ambient lighting. A Nikon D3400 digital SLR camera was positioned 18 cm from the plate at a 40-degree downward angle. The camera’s flash was turned on to create a reflection across the surface, which emphasized where the color was spreading. The lighting was arranged to avoid glare while capturing the surface of the milk, allowing the color to stand out clearly against the white background.
The image was captured using a Nikon D3400 digital single-lens reflex camera with a 55 mm focal length. The field of view was approximately 25.4 cm, and the distance from the camera to the subject was 18 cm. The exposure settings were an aperture of f/5.6, a shutter speed of 1/60 second, and an ISO of 4000. The original image dimensions were 6000 × 4000 pixels, and the final edited image was cropped to 5016 × 3296 pixels. Post-processing was completed in GIMP, where the contrast was increased to highlight the edges of the flow patterns and the color balance was adjusted by enhancing the magenta and cyan highlights. These edits helped bring out contrast and emphasized the variations in surface flow caused by the soap’s interaction with the milk’s fat content. The original image is shown below.
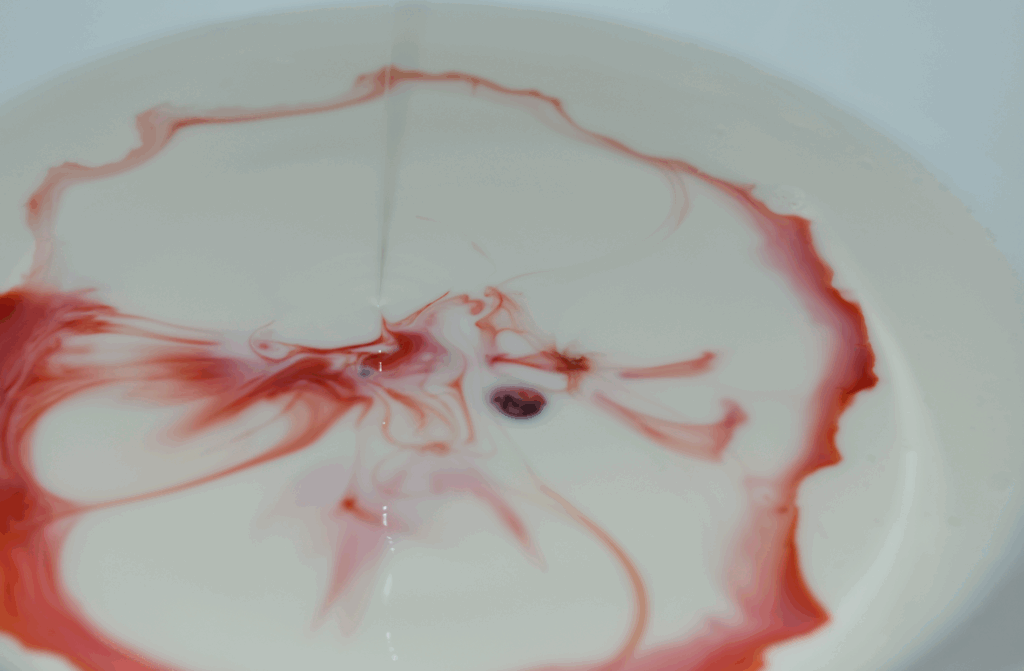
Figure 2: Original Image of Phenomenon
The final image captures the disruption of surface tension in milk caused by the surfactant action of soap. The food coloring clearly outlines the flow directed by surface tension gradients, forming circular and branching shapes. I like that the image was taken just microseconds after the soap was dropped, allowing the viewer to see fluctuations across the surface. One aspect I would improve is reducing image noise caused by the high ISO setting, which caused graininess. The fluid physics show how surfactants create motion in a still liquid by altering intermolecular forces. If I were to extend this work, I would look into how different types of surfactants or differing milk fat percentages affect the speed and pattern of the resulting flows. Overall, the image fulfilled my intent by capturing an artistic example of surface tension dynamics that is both visually and scientifically engaging.
Appendix
A Scientific Soap Opera, Starring the Marangoni Effect (2018) UCSB College of Engineering. Available at: https://engineering.ucsb.edu/news/scientific-soap-opera-starring-marangoni-effect.
LECTURE 4: Marangoni flows (no date). Available at: https://web.mit.edu/1.63/www/Lec-notes/Surfacetension/Lecture4.pdf.


4 Comments. Leave new
Nice visualization of miscible mixing. You can clearly see the diffusion and the early-stage instabilities in the dye.
This has very impressive colors, the editing and cropping did a great job bringing out eh phenomenon and preventing distractions
Hi Cooper, I really love the colors you chose for this experiment. I also think that it’s really interesting that it spread into a near circle, and how most of the dye spread to the left. Really interesting pattern here, nice work!
Your editing is fantastic! I like the black voids, and it brings out contrast in the areas with less dye concentration.